Patent Owner: Sobei Genomics, Inc
Technical fields:
Summary
The present invention relates to the anti-aging application of telomerase reverse transcriptase that increases the activity of telomerase in cells. Such compositions contain formulations of drugs (including local) and nutritional supplements. The methods and compositions are used to treat diseases, such as HIV infection, various degenerative diseases, and acute or chronic skin nutrition products (aliments) by increasing the activity of telomerase in cells or tissues of patients. They can also be used to increase the replication capacity of cultured cells, such as in vivo cell therapy and the proliferation of stem cells.
The present invention provides novel telomerase genes and proteins and relates to the cloning and characterization of the catalytic protein component of telomerase, termed telomerase reverse transcriptase ("TRT").
In particular, the present invention relates to telomerase isolated from Euplotes aediculatus, two polypeptide subunits of this telomerase of 123 and 43 kilodaltons, and polypeptides, nucleic acids and sequences of homologs of E. aediculatus telomerase in fission yeast, other yeast, Tetrahymena, other fungi, mice and other mammals.
Telomerase reverse transcriptase compositions and methods for increasing telomerase activity
Invention Field
The present invention relates to methods and compositions for inducing telomerase activity in cells.
Technical Background and References
Telomerase
Telomerase is a ribonucleoprotein that catalyzes the addition of telomere repeats at the ends of telomeres. Telomeres are long sequences of repetitive sequences that cap the ends of chromosomes and are thought to stabilize chromosomes. In humans, telomeres are typically 7-10 kb in length and include multiple repeats of the sequence -TTAGGG-. Telomerase is not expressed in the vast majority of adult cells, and with successive cycles of replication, telomere length decreases. After a certain number of replication cycles, the progressive shortening of telomeres causes cells to enter a critical stage of telomere loss, which in turn leads to cellular senescence. Certain diseases are associated with rapid telomere shortening, leading to premature cellular senescence. Expression of the gene encoding the human telomerase protein in human cells has been shown to confer an immortal phenotype, presumably bypassing the natural aging pathway of the cell. Furthermore, expression of the telomerase gene in aged cells that were shown to have short telomeres resulted in increased telomere length and preservation of a phenotype typically associated with young cells.
In contrast to tumor cells and certain stem cells, somatic cells have little or no telomerase activity and cease to divide when the telomere ends of at least some chromosomes shorten to a critical length, leading to programmed cellular senescence (cell death). Since the loss of telomeric repeat sequences that leads to senescence in somatic cells is increased by low telomerase activity, the induction of telomerase activity (which has the effect of adding large amounts of telomeric repeat sequences to telomeres) can give dying cells increased replicative capacity, give senescent cells the ability to proliferate, and appropriately allow damaged tissues to exit the cell cycle once they have been repaired. [0006] Potential therapeutic advantages of increasing telomerase activity in somatic cells include, for example, AIDS treatment (characterized by premature senescence of cytotoxic T lymphocytes (CD8+ cells) whose role is to kill infected CD4' cells) (see, Dagarag et al., 2003); neuroprotection in Alzheimer's patients (see, Mattson et al., 2000); wound healing and maintenance of cells such as the adrenal cortex (see, Thomas et al., 2000) or transplantation of bone marrow or stromal/interstitial cells (see, Simonsen et al., 2002).
Invention content
Generally, the present invention relates to methods for increasing telomerase activity in cells and compositions for use in the methods. The methods and compositions can be used for cells in cell culture (i.e., cultured in vitro or indirectly in vivo, or directly in vivo), such as cells grown on tissues in vivo (including humans and non-human animals, especially non-human mammals).
In particular embodiments, the composition includes a compound having the following general formula I, II or II. Aspects of the invention include formulations of such compounds for use in cosmetics, nutritional supplements and pharmaceutical applications, particularly in applications where increased telomerase activity in cells is shown, or is expected to be shown, to be beneficial. Methods of using the compounds and formulations thereof in such applications are also provided, including methods of applying or administering such formulations after determining that it is necessary or beneficial to increase telomerase activity in cells or tissues.
The present invention also includes, in one aspect, a method for increasing telomerase activity in a cell or tissue. The method comprises contacting the cell or tissue with a formulation of an isolated compound having the following general formula I, II or II. In a preferred embodiment, the compound is of the following general formula I or II. The method further comprises a pre-step of determining that the telomerase activity in the cell or tissue needs to be increased.
In compounds having the general formula I:
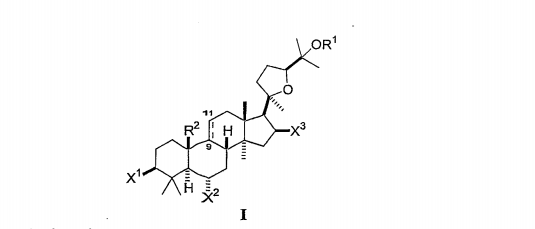
Each of X¹, X² and X² is independently selected from hydroxy, lower alkoxy, lower acyloxy, keto or glycoside;
OR' is selected from hydroxy, lower alkoxy, lower acyloxy, or glycoside;
wherein any hydroxyl group on the glycoside may be substituted by other glycosides, lower alkyl groups or lower acyl groups so that the compound contains up to three glycosides;
and R² is methyl and = indicates a double bond between carbons 9 and 11; or R² together with carbon 9 form a fused cyclopropyl ring and = indicates a single bond between carbons 9 and 11.
Preferably, the compound comprises 0, 1 or 2, more preferably 0 or 2 glycosides, none of which are substituted by other glycosides. Preferably, each of the glycosides is in the D (naturally occurring) configuration.
In selected embodiments of Formula I, each X¹ and X² is independently selected from hydroxyl, lower alkoxy, lower acyloxy, or glycoside; and X³ is independently selected from hydroxyl, lower alkoxy, lower acyloxy, keto, or glycoside. In further embodiments, X¹ is OH or glycoside, each X² and OR' is independently OH or glycoside, and X² is OH or keto. Exemplary compounds of Formula I include astragaloside IV, cycloastragenol, astragenol, astragaloside IV 16-one, cycloastragenol 6-β-D-pyranose glucoside, and cycloastragenol 3-β-D-pyranose glucoside. In selected embodiments, the compound is selected from astragaloside IV, cycloastragenol, astragenol, or astragaloside IV 16-one. In one embodiment, the compound is astragaloside IV.
In the compound having the general formula II:
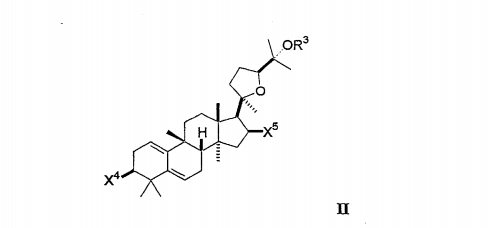
Each X* and X⁵ is independently selected from hydroxyl, lower alkoxy, lower acyloxy, keto or glycoside, and [0086] OR³ is selected from hydroxyl, lower alkoxy, lower acyloxy, or glycoside,
Any hydroxyl group on the glycoside may be substituted by other glycosides, lower alkyl groups or lower acyl groups so that the compound contains up to three glycosides.
Preferably, the compound comprises 0, 1 or 2 glycosides, none of which are substituted by other glycosides; each of the glycosides is optionally in the D configuration.
In the selected embodiment of the general formula II, each X and OR³ is independently selected from hydroxyl, lower alkoxy, lower acyloxy or glycoside; and X⁵ is selected from hydroxyl, lower alkoxy, lower acyloxy or keto (=0). In a further embodiment, X* is OH or glycoside, and each X⁵ and OR³ is OH. In one embodiment, x* is OH. [0090] In the compound of the general formula IⅡ:
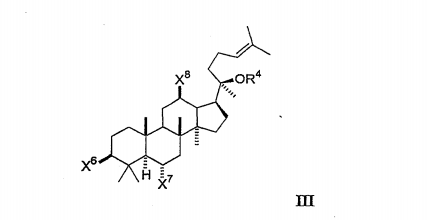
Each of X⁶, X⁷ and X⁸ is independently selected from hydroxyl, lower alkoxy, lower acyloxy, keto or glycoside, and OR* is selected from hydroxyl, lower alkoxy, lower acyloxy, or glycoside, [0094] wherein any hydroxyl group on the glycoside may be substituted by other glycosides, lower alkyl or lower acyl groups so that the compound contains up to three glycosides.
Preferably, the compound comprises 0, 1 or 2 glycosides, none of which are substituted by other glycosides; each of the glycosides is in the D configuration.
Each of x⁶, x⁷ and x⁸ is independently selected from hydroxyl, lower alkoxy, lower acyloxy, keto or glycoside, and [0093] OR* is selected from hydroxyl, lower alkoxy, lower acyloxy, or glycoside,
Any hydroxyl group on the glycoside may be substituted by other glycosides, lower alkyl groups or lower acyl groups so that the compound contains up to three glycosides.
Preferably, the compound comprises 0, 1 or 2 glycosides, none of which are substituted by other glycosides; each of the glycosides is in the D configuration.
In the selected embodiment of formula II, each x⁶, X', x⁸ and OR* is independently selected from hydroxyl, lower alkoxy, lower acyloxy or glycoside, preferably hydroxyl and glycoside. In a further embodiment, each x⁸ and OR² is OH, and each x⁶ and X² is independently selected from hydroxyl or glycoside. In a further embodiment, each OR², X⁸ and X⁸ is OH, and X² is glycoside. An exemplary compound of formula III is ginsenoside RH1.
When the preferred compound of the above general formula I, I or III is dissolved in a solvent at a concentration of 1 μg/ml or less for formulation, the compound is effective in producing a level of telomerase activity (measured by the TRAP method) in keratinocytes or fibroblasts, which level is at least 50% higher than the level in the cells treated with the solvent alone (measured by the TRAP method described herein). In a further preferred embodiment, the compound is effective in producing a level of telomerase activity (measured by the TRAP method) in keratinocytes or fibroblasts, which level is at least 100% higher than the level in the cells treated with the solvent alone.
Exemplary compounds of Formula I-III include compounds depicted in FIG. 1 and designated herein as 1 (astragaloside IV), 2 (cycloastragenol), 3 (astragenol), 4 (astragaloside IV 16-one), 5 (20R,24S-epoxy-3β,16β,25-trihydroxy-9β-methyl-19-norlanost-1,5-diene), 6 (cycloastragenol 6-β-D-pyranoglucoside), 7 (cycloastragenol 3-β-D-pyranoglucoside) and 8 (ginsenoside RH1). In selected embodiments, the compound is selected from 1 (astragaloside IV), 2 (cycloastragenol), 3 (astragenol), 4 (astragaloside IV 16-one), 5 (20R, 24S-epoxy-3β, 16β, 25-trihydroxy-9β-methyl-19-norlanost-1,5-diene), 6 (cycloastragenol 6-β-D-pyranoglucoside) or 7 (cycloastragenol 3-β-D-pyranoglucoside) as specified herein. In further embodiments, the compound is selected from 1, 2, 3, 4, or 5 as specified herein. In one embodiment, the compound is astragaloside IV (1) or cycloastragenol (2).
The method of contacting a formulation of an isolated compound of Formula I, II or III with a cell or tissue may include, prior to said contacting, identifying a cell or tissue in need of increased telomerase activity. Advantages achieved by increasing telomerase activity in a cell or tissue include, for example, an increase in replication capacity and/or an extension of the life of the cell or cells in said tissue. [0099] The method may include diagnosing a condition in which a subject is in need of increased telomerase activity in cells or tissues, and administering the formulation to the subject. Preferably, the subject is a mammalian subject, such as a human subject or patient. Such conditions may include, for example, HIV infection, various degenerative diseases such as neurodegenerative diseases, bone or joint degenerative diseases, macular degeneration, atherosclerosis or anemia. Such conditions also include trauma or other acute or chronic diseases of the epidermis, such as burns, abrasions, cuts, dislocations, injuries caused by infectious agents, chronic varicose ulcers, diabetic ulcers, pressure ulcers, bedsores, mucosal ulcers, or keloid formations.
Thus, the present invention provides a method of treating a patient suffering from a disease as described above by increasing telomerase activity in cells or tissues of the patient, said method comprising administering to a patient in need of such treatment a formulation of an isolated compound of formula I, formula II or formula III as defined above. The composition may be administered by a variety of routes, such as orally, topically or parenterally. [0096] The present invention further provides a method of diagnosing a subject suffering from a disease state, allowing it to be treated by increasing telomerase activity in cells or tissues of the subject and administering to the subject in need of said treatment a compound of formula I, III or III, preferably a compound of formula I or II, in a pharmaceutical carrier.
In another aspect, the present invention provides a method for treating acute or chronic diseases of the epidermis comprising contacting epidermal cells with a topical formulation of an isolated compound of Formula I, Formula II or Formula III as described above. In preferred embodiments, the compound is of Formula I or Formula II. In further embodiments, the compound is selected from TA-65, cycloastragenol, astragaloside IV 16-one, cycloastragenol 6-β-D-pyranoglucoside, cycloastragenol 3-β-D-pyranoglucoside and 20R,24S-epoxy-3β,16β,25-trihydroxy-9β-methyl-19-norlanost-1,5-diene (designated herein as 5).
Cells contacted with the formulation may also include transplanted cells contacted in vivo, such as for cell-based therapy, or other cells in culture. Therefore, the present invention provides a method for enhancing the replication capacity of cells in vitro or in vivo, comprising contacting the cells with an effective amount of a composition containing the above-mentioned general formula I, general formula I or general formula III (including the compounds in the above-mentioned selected embodiments). In a preferred embodiment, the compound is a compound of general formula I or general formula II in the above-mentioned selected embodiments. Typically, the cell is a non-transformed mammalian cell; in selected embodiments, the cell is a stem cell, such as a bone marrow stem cell, a bone marrow stromal cell, a young or early passage skin fibroblast, a pancreatic islet precursor cell, a glomerular cell, a renal cortical cell, a myosatellite cell, an osteoblast, a retinal pigment epithelial cell, and an HIV-restricted CD8* cell.
In a related aspect, the present invention provides a pharmaceutical composition comprising a compound of formula I as described above in a pharmaceutically acceptable carrier, wherein:
Each X', X² is independently selected from hydroxyl, lower alkoxy, lower acyloxy, keto, or glycoside; [0107] X₃ is keto;
OR' is selected from hydroxy, lower alkoxy, lower acyloxy, or glycoside;
Wherein any hydroxyl group on the glycoside can be substituted by other glycosides, lower alkyl or lower acyl groups so that the compound contains up to three glycosides; and R² is methyl and di represents a double bond between carbons 9 and 11; or in a preferred embodiment R² forms a fused cyclopropyl ring with carbon 9, and di represents a single bond between carbons 9 and 11.
Preferably, the compound comprises 0, 1 or 2 glycosides, none of which are substituted by other glycosides, and each of the glycosides is in the D configuration.
In selected embodiments, each X¹ is OH or glycoside, and each X² and OR¹ is independently OH or glycoside.
In one embodiment, the compound is astragaloside IV 16-one (designated herein as 4).
Alternatively, the composition comprises, in a pharmaceutically acceptable carrier, a compound of formula I as described above, wherein:
One of X¹ and X² is selected from hydroxyl, lower alkoxy, lower acyloxy, or keto, and the other is a glycoside; [0115] Each of X₃ and OR' is independently selected from hydroxyl, lower alkoxy, lower acyloxy, or glycoside;
Wherein any hydroxyl group on the glycoside can be substituted by other glycosides, lower alkyl or lower acyl groups so that the compound contains up to three glycosides; and R² is methyl and di represents a double bond between carbons 9 and 11; or in a preferred embodiment R² forms a fused cyclopropyl ring with carbon 9, and = represents a single bond between carbons 9 and 11.
Preferably, the compound comprises 1 glycoside which is not substituted by other glycosides, and the glycoside is in the D configuration.
In one embodiment, the compound is selected from cycloastragenol 6-β-D-glucopyranoside (designated herein as 6) or cycloastragenol 3-β-D-xylopyranoside (designated herein as 7).
Alternatively, the pharmaceutical composition comprises a compound of formula II as described above in a pharmaceutically acceptable carrier. Selected embodiments of the compound are also as defined above. In one embodiment, the compound is 5 as specified herein.
The present invention also provides compounds of formula II as described above, including selected embodiments as described above. In one embodiment, the compound is designated 5 herein. [0121] In a related aspect, the present invention provides topical pharmaceutical formulations of isolated compounds of formula I, formula III or formula III as described above. Selected embodiments of the compounds are also as defined above. In preferred embodiments, the compound is of the following formula I or II. In a further embodiment, the compound is selected from astragaloside IV, cycloastragenol, astragenol, astragaloside IV 16-one, cycloastragenol 6-β-D-pyranose glucoside, cycloastragenol 3-β-D-pyranose glucoside and 20R,24S-epoxy-3β,16β,25-trihydroxy-9β-methyl-19-norlanost-1,5-diene (designated 5 herein). Topical formulations typically include one or more ingredients selected from emulsifiers, thickeners or emollients. Such compositions can be used for the treatment of wounds or other acute or chronic diseases of the epidermis.
In another related aspect, the present invention provides a nutritional supplement composition comprising a nutritional supplement formulation of an isolated compound of Formula I, Formula I or Formula III as described above. The selected embodiments of the compound are also as defined above. In a preferred embodiment, the compound is selected from astragaloside IV, cycloastragenol, astragenol, astragaloside IV16-one, cycloastragenol 6-β-D-pyranose glucoside, cycloastragenol 3-β-D-pyranose glucoside and 20R,24S-epoxy-3β,16β,25-trihydroxy-9β-methyl-19-norlanost-1,5-diene (designated herein as 5). In a further embodiment, in addition to the isolated compound of formula I, III or II, the nutritional supplement formula further comprises a nutritional supplement herbal extract, which may be an extract of Astragalus membranaceus.
The isolated compounds of the general formula I, II or III including the selected embodiments described above can also be used for the preparation of medicaments for treating diseases that can be treated by increasing telomerase activity in cells or tissues. Examples of such diseases are discussed in detail below. Similarly, the isolated compounds of the general formula I, II or II including the selected embodiments described above can also be used for the preparation of medicaments for the treatment of acute or chronic diseases of the epidermis. In the preferred embodiments of the above-mentioned uses, the isolated compound has the following general formula I or II, including the selected embodiments of the general formula I or II described above.
The present invention also provides a method for selecting a compound effective in increasing intracellular telomerase activity. According to this method, the derivatives of the compounds of Formula I, Formula II, and Formula II as described above are tested for their ability to increase telomerase activity in keratinocytes or fibroblasts, as determined by the TRAP method described herein. The derivative can be selected when the derivative is formulated in a solvent and used at a concentration of 1 μg/ml or less and is effective in producing a certain level of telomerase activity in keratinocytes or fibroblasts (as determined by the TRAP method), which level is at least 50% higher than the level in the cells treated with the solvent alone, preferably at least 100% higher. The derivative can then be formulated with a carrier for topical, pharmaceutical or nutritional supplementation.
In a related aspect, the present invention also provides a method for selecting an agent for treating acute or chronic diseases of the epidermis. According to this method, the wound healing ability of the derivatives of the compounds of general formula I, general formula II, and general formula III as described above in keratinocytes or fibroblasts is tested using a (skin) scratch test method, and if the wound healing activity of the derivative at a concentration of 1 μg/ml or less than 1 μg/ml is at least 50%, preferably at least 100%, higher than that of the control treated with the control solution, the derivative can be selected. The derivative can then be formulated with a topical carrier.