Telomeres are the terminal structures of linear chromosomes in eukaryotic cells. They play a protective role in the process of cell replication, preventing DNA damage, and effectively prevent inter-chromosomal terminal recombination, fusion and chromosome degradation like a cap.
During the process of cell mitosis, telomeres gradually shorten as the number of cell divisions increases. When telomeres shorten to a certain extent, they can no longer maintain the stability of chromosomes, leading to cell dysfunction and even death. Therefore, telomere shortening is also considered a sign of aging, and telomerase can prevent telomere shortening.
As in humans, the zebrafish gut is one of the organs with the fastest telomere shortening, which leads to early tissue dysfunction in both normally aged zebrafish and zebrafish with telomerase mutants that cause premature aging. However, whether telomere-dependent aging of a single organ (the gut) leads to systemic aging is still unclear.
On May 4, 2023, Professor Miguel Godinho Ferreira's team from the Institute of Cancer and Aging in Nice, France, published an article titled "Gut-specific telomerase expression counteracts systemic aging in telomerase-deficient zebrafish" in the journal Nature Aging.
The study found that tissue-specific telomerase expression in the intestine can prevent telomere shortening and rescue tert-/-mediated premature aging, and can extend the lifespan of tert-/- zebrafish by 40% while improving natural aging.

To investigate how telomere-dependent intestinal aging affects organisms, the researchers constructed a zebrafish transgenic line containing a Cre-inducible zebrafish tert transgene driven by the intestinal cell-specific fabp2 promoter in a tert +/- genetic background.
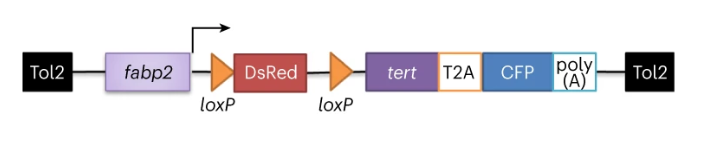
To test whether the expression of the tert transgene can prevent telomere shortening, the researchers performed telomere restriction fragment (TRF) analysis on intestinal samples of 9-month-old zebrafish and found that the telomere length of blood cells in the tert knockout group was significantly reduced compared with the control group. Although the expression of tert complement DNA (cDNA) driven by the fabp2 promoter did not restore telomere length to WT levels, the induction of the tert transgene also significantly extended the telomere length of zebrafish in the tert knockout group.
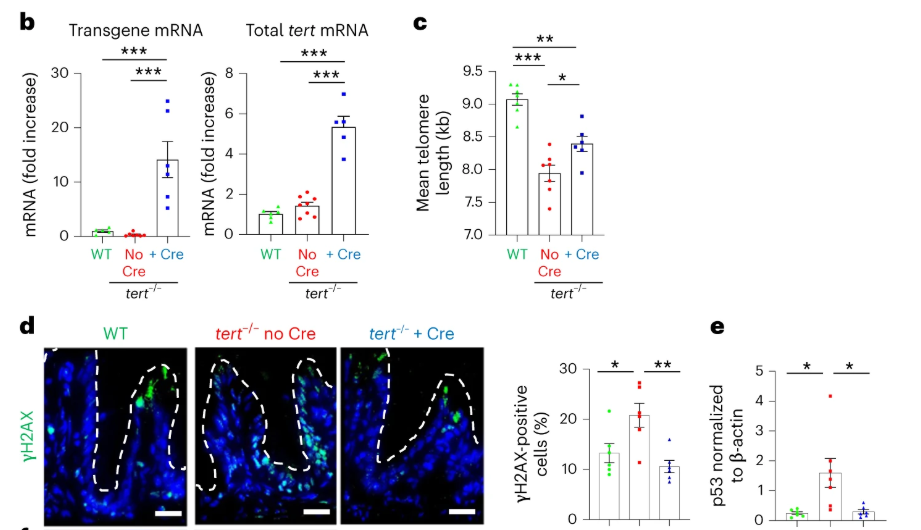
Subsequently, through transcriptomics and metabolomics analysis, it was determined that telomerase expression can rescue the systemic aging symptoms of tert knockout zebrafish. Specifically, telomerase expression can improve intestinal flora imbalance, systemic tissue degeneration, and hematopoietic system aging.
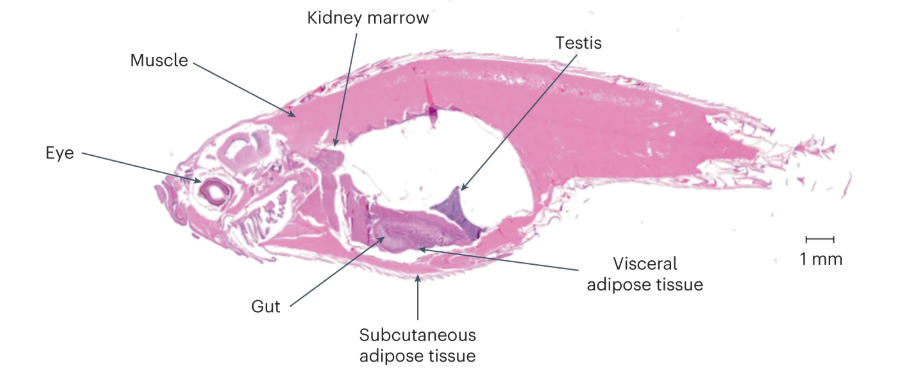
The researchers then tested whether the expression of telomerase in the intestine would affect the lifespan of zebrafish and found that the telomeres of zebrafish in the tert knockout group were shortened and the average lifespan was shortened to 12-18 months, while the average lifespan of zebrafish in the control group was over 42 months.
Notably, delaying intestinal aging extended the average lifespan of tert knockout zebrafish by approximately 40%. However, telomerase expression in the intestine was not sufficient to fully extend lifespan to healthy control levels, suggesting that telomere shortening in other organs may also be limiting at a later stage.
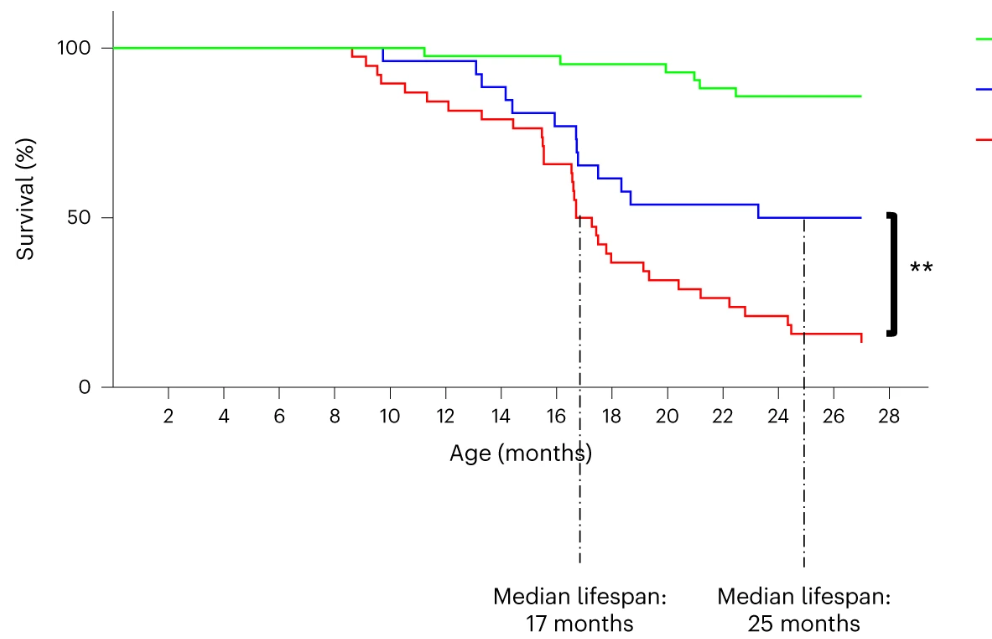
In summary, this study demonstrates that gut-specific telomerase expression in tert-knockout zebrafish delays gut aging, which in turn improves the health of the entire organism, including improving gut microbiota dysbiosis and delaying aging of multiple organs. Therefore, gut telomere-dependent aging controls aging of the entire organism.
Reference: https://www.nature.com/articles/s43587-023-00401-5