
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the cause of the 2019 novel coronavirus (COVID-19) epidemic. Recent studies have highlighted the important role of the human angiotensin-converting enzyme 2 (ACE2) molecule in mediating the cellular entry of SARS-CoV-2. Binding to the ACE2 receptor is a key initiation step for SARS-CoV-2 to infect target cells and is critical for human infection, and the expression and distribution of the ACE2 receptor may be associated with the progression and prognosis of COVID-19.
Telomeres are regions at the ends of linear chromosomes, and vertebrate telomeres are composed of a large number of typical TTAGGG repeat sequences. The shelterin protein complex binds to telomeric DNA and protects the telomeric DNA from being recognized as DNA damage (DD), thereby preventing the DDR (DNA damage response). DNA replication causes the telomeres of chromosomes to gradually shorten. When telomeres become very short, they are sensed as DNA double-strand breaks (DSBs) and activate the DDR pathway. Continuous inbreeding in mice leads to progressive telomere shortening and DDR activation at telomeres, and accumulates characteristics of aging and aging-related diseases. In addition, because damage at telomeres is not easily repaired, this leads to persistent DDR activation. In this article, Professor Fabrizio d'Adda di Fagagna and his team from the Institute of Molecular Genetics of the National Research Council in Pavia, Italy, aimed to better characterize the regulation of ACE2 expression during aging following telomere shortening and DDR activation.
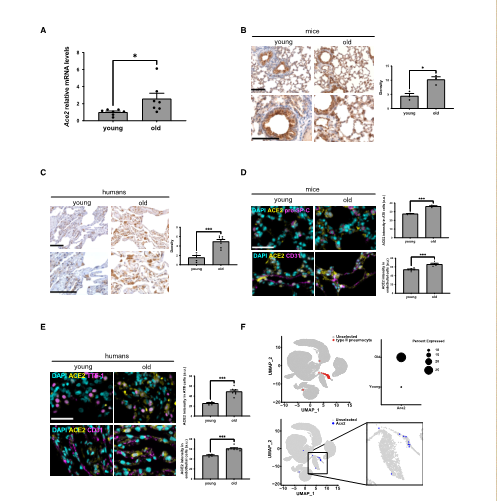
To understand the regulation of ACE2 expression during aging, Professor Fabrizio d'Adda di Fagagna and his team from Italy studied the expression of ACE2 in the lungs of mice and humans of different ages. By RT-qPCR detection and immunohistochemistry (IHC), it was observed that the levels of ACE2 mRNA and protein in the lungs of old mice (22-24 months) increased compared with young mice (2-3 months). Similarly, in human lungs, it was found that the expression level of ACE2 protein in elderly subjects was higher than that in young subjects. To confirm that ACE2 increased in ATII cells, the team performed double-labeled immunofluorescence detection of ACE2 in lung samples of different ages and found that the expression of ACE2 in ATII lung cells increased with age, whether in mice or humans. The team further studied the differential expression of ACE2 transcripts and observed that ACE2 increased with age in ATII lung cells, while the housekeeping gene GAPDH was widely expressed in almost all cell types and did not change in the analyzed age groups. In summary, it can be demonstrated that when cells age, the expression of ACE2 increases, and the increase in ACE2 expression is mainly in ATII lung cells. (Figure 1)
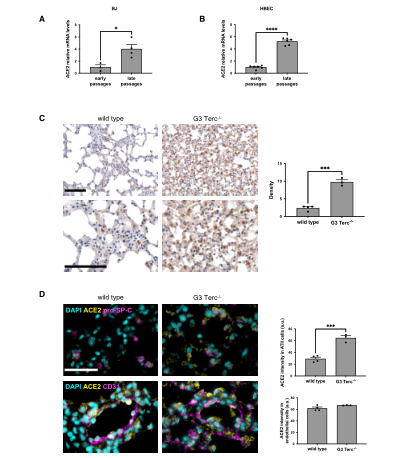
Next, to reveal the molecular mechanisms that control ACE2 upregulation during aging, the team conducted in vitro and in vivo experiments. To test whether telomere shortening is sufficient to regulate ACE2 expression, the team measured ACE2 mRNA levels in human fibroblasts (BJ) and human bronchial epithelial cells (HBECs) from different populations. Because both cell types lack telomere maintenance mechanisms and progressively shorten telomeres during proliferation. It was found that ACE2 mRNA levels in late-passage BJ and HBEC were increased compared with early-passage cells. Next, the team expanded the study to a mouse model lacking telomerase RNA components. In the analysis of the third generation of mice lacking telomerase RNA, it was found that compared with wild-type animals matched for age and sex, mice lacking telomerase RNA had a continuous increase in ACE2 expression, and immunofluorescence imaging showed that ACE2 was mainly increased in ATII lung cells expressing ACE2. The same phenomenon was also observed in pro-SP-C cells. These results clearly show that telomere shortening plays a role in regulating ACE2 levels in human cells and mouse tissues. (Figure 2)
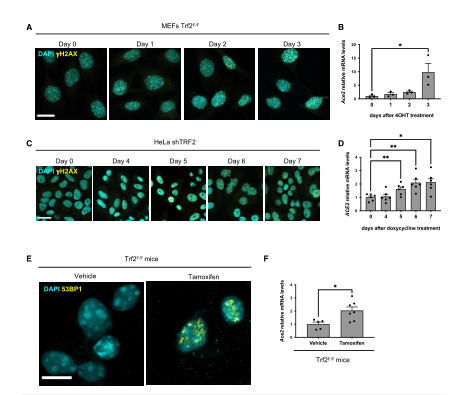
Because when telomeres become very short, they activate the DNA damage response (DDR) pathway. So next the team tested whether the telomeric DDR was sufficient to increase ACE2 mRNA levels, the team used two mammalian cell systems that allow for specific activation of the DDR at telomeres in the absence of telomere shortening. After knocking out TRF2, the team found an increase in H2AX expression to demonstrate activation of the DDR pathway. The increase in DDR expression was accompanied by an increase in ACE2 mRNA levels, and the same results were observed after ionizing radiation (IR) in the same cell line, demonstrating that the DDR signaling pathway plays a role in increasing ACE2 mRNA levels. To verify the role of telomere DDR in vivo, the team administered tamoxifen to mice to induce TRF2 expression loss, activate the DDR pathway at the telomeres, and observed an increase in ACE2 mRNA levels in mouse liver. This also confirmed the above results. In summary, these results in human and mouse cell lines and mouse tissues indicate that the activated DDR pathway has a conservative role in regulating ACE2 levels. Since telomere DDR accumulates during the aging process, this may lead to increased ACE2 levels. (Figure 3)
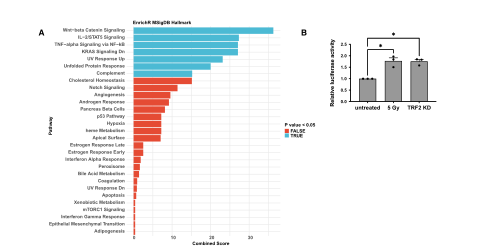
The team believes that the increase in ACE2 mRNA levels is caused by the enhanced activity of its transcriptional promoter. To determine whether the ACE2 promoter responds to DDR activation, the team performed in silico analysis to identify transcription factors whose DNA binding motifs are significantly enriched in the ACE2 promoter region. The results showed that among these transcription factors, there are pathways related to the DNA damage response. The team transfected HeLa shTRF2 cells with a plasmid carrying a luciferase reporter gene under the control of the human ACE2 promoter and induced telomeric DDR by TRF2 knockout, and found that the luciferase signal increased. And similar transcriptional activation was also observed when DDR was activated in irradiated uninduced HeLa cells. This suggests that the DDR pathway increases ACE2 mRNA levels by controlling ACE2 promoter activity. (Figure 4)
To further prove that key components of the DDR pathway are responsible for the observed increase in ACE2 mRNA levels, the team used the ATM kinase inhibitor KU-60019 (ATMi) to prevent the formation of DDR lesions. It was found that the increase in ACE2 mRNA levels was significantly reduced, indicating that ATM kinase activity is involved in the regulation of ACE2 transcription levels. To investigate whether the inhibition of telomeric DDR affects the expression of ACE2 in vivo, the team used telomeric antisense oligonucleotides (tASOs) to achieve specific inhibition of telomeric DDR. When the mice were sacrificed and their tissues were analyzed, it was found that both tASO treatments were very effective and ACE2 mRNA levels were downregulated in vivo. Next, the team expanded the study to the third generation of mice with shortened telomeres, and found that ACE2 expression was significantly reduced after treatment with tASOs or tASOs by double-label immunostaining. These results support that ACE2 regulation is caused by subsequent DDR activation rather than telomere shortening. (Figure 5)
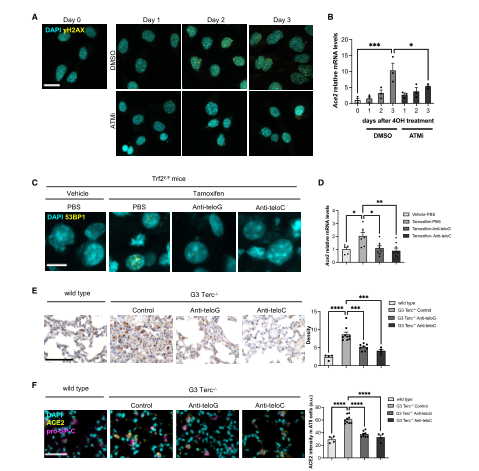
Taken together, these results suggest that expression of the SARS-CoV-2 receptor ACE2 is directly regulated by activation of the DDR pathway at the transcriptional level and that telomere dysfunction is a physiological event that can participate in the DDR pathway in regulating ACE2 levels.
Original link: https://www.embopress.org/doi/full/10.15252/embr.202153658