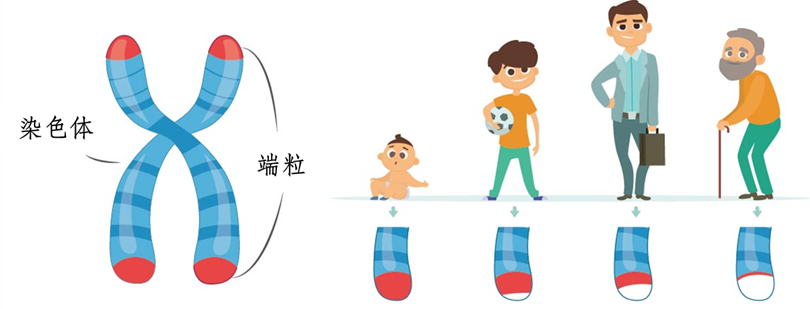
Telomeres are the "caps" at both ends of chromosomes , like the plastic tips at the end of shoelaces, protecting chromosomes from degradation and unraveling. As we age, the number of cell divisions increases, and telomeres gradually consume and shorten. When telomeres are so short that they can no longer protect chromosomes from damage, the genome becomes unstable and cells initiate a silent or death program . This program kills cells with unstable telomeres and cancer-related chromosomal aberrations. Such cells tend to escape the proliferation restrictions controlled by telomeres and achieve "immortality" (cancer). Before " exhaustion ", telomeres will " communicate " with the cell's " power plant " (mitochondria) , and through a " nuclear explosion " (inflammatory response), they will initiate a " self-destruction " program to kill cells that may become cancerous.
Telomeres: My length, the years of protecting cells are good
Telomeres are located at the ends of eukaryotic cell chromosomes and together with telomere-binding proteins form a special "cap-like" structure that maintains the integrity of chromosomes and controls the cell division cycle. They are essentially a small DNA-protein complex. According to the mechanism and characteristics of DNA replication, after each chromosome replication, a small segment of the DNA end on the extended chain cannot be replicated. Therefore, each time a cell divides, the telomere shortens by 30 bp-200 bp. When it shortens to a specific critical value of 2 kb-4 kb (crisis length), the cell will enter a silent period or initiate apoptosis. Therefore, telomere length has a significant relationship with cell aging [1].
However, some cells in the human body, such as spermatogonia and tumor cells, contain telomerase, which can attach new telomere fragments to the ends of DNA, so that the telomeres remain at a stable length and do not shorten with the increase in the number of divisions, thereby achieving rapid cell proliferation and immortality. Compared with normal somatic cells, the telomeres of tumor cells are generally shorter. Although telomerase activity can be detected in 85%-90% of tumors, telomerase is not the direct cause of cell canceration, but plays a decisive role in the growth and proliferation of tumors. Therefore, telomerase activation occurs after canceration (when the first tumor cell has been generated), and telomerase makes tumor cells immortal and able to divide and proliferate indefinitely [2].
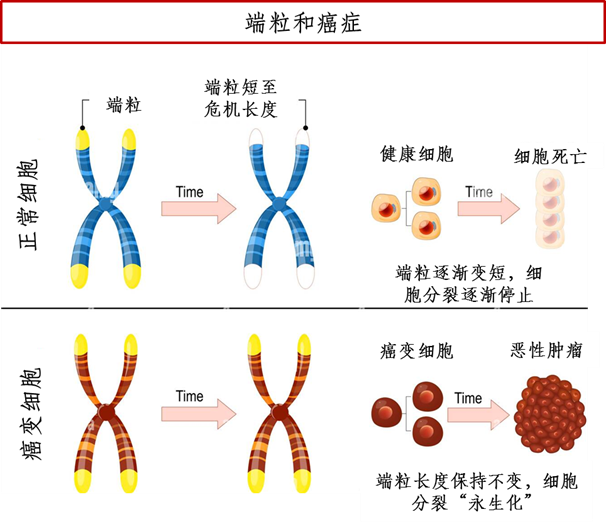
Figure 1 A simplified diagram of the relationship between telomeres and cancer (Drawing: BioExploration Editorial Team)
The 2009 Nobel Prize in Physiology or Medicine was awarded to three scientists, including Elizabeth Helen Blackburn, for their outstanding contributions to the research of telomeres and telomerase, in recognition of their discovery of the mechanism by which telomeres and telomerase protect chromosomes. Since then, the research on telomeres and telomerase has received increasing attention in the field of cancer, especially the mechanism of blocking telomere extension and telomerase inhibitors, which may become potential new targets for anti-cancer treatment [3].
How do telomeres prevent cancer formation?
The shortening of telomere length increases the genetic instability of cells, making it easy for abnormal conditions such as gene mutations to occur, increasing the risk of tumors. Once tumor cells are generated, the action of telomerase causes the telomeres to be "abnormally" stabilized at a certain length, achieving immortality. To maintain the body's balance, telomeres need to find a balance between natural shortening and stability to prevent the formation of cancer. So, how do telomeres control this balance?
In2019, Jan Karlseder's research team at the Salk Institute for Biological Studies in California, USA, discovered that cells with telomeres at a critical length are cleared through a process called autophagy, in which the body clears damaged cells on its own. This beneficial natural process removes cells with very short telomeres and unstable genomes and is a powerful barrier against cancer formation [4]. However, it remains unknown how the autophagy-dependent cell death program is activated during the crisis period when telomeres are extremely short.
In 2023, Jan Karlseder's team, who studies telomere biology and how telomeres prevent cancer formation, teamed up with Gerald Shadel's team, who studies the role of mitochondria in human disease, aging, and the immune system, to explore the above questions. Using human skin fibroblasts for genetic screening, they discovered an interdependent immune-sensing and inflammatory signaling pathway (similar to the immune system's antiviral pathway) that is critical for cell death during crisis. Specifically, they found that RNA molecules from short telomeres activated immune sensors on the outer surface of mitochondria called ZBP1 and MAVS in a unique way.
Figure 2 Research results (Source: [5])
When telomeres become critically short, they communicate with the cell’s “power plants” – the mitochondria. This communication triggers a complex set of signaling pathways and an inflammatory response that damages cells that may become cancerous. These findings demonstrate an important link between telomeres, mitochondria, and inflammation, and highlight how cells can bypass the critical length barrier and become cancerous when pathways do not function properly. This could lead to new ways to prevent and treat cancer, as well as to design better interventions to counteract the harmful consequences of aging[5].
"Telomeres, mitochondria and inflammation are three hallmarks of aging that have most often been studied separately," said Shadel. "Our results reveal a telomere-mediated mechanism of tumor suppression, showing that stressed telomeres send RNA messages to mitochondria to induce inflammation. This highlights the need to study the interplay between these hallmarks to fully understand aging and potentially intervene to extend human healthspan."
Joe Shadel, a senior researcher in Jan Karlseder's group , "Cancer development is not a simple process, but rather a multistep one that requires many alterations and changes throughout the cell. A better understanding of the complex pathways connecting telomeres and mitochondria may lead to the development of novel cancer therapies in the future," said Nassour.
There is a synergy between critically short telomeres, mitochondria, and innate immunity that has evolved to protect against age-related cancers in humans. Next, the scientists plan to further investigate the molecular basis of these pathways and explore the therapeutic potential of targeting them to prevent or treat cancer.